

| Tracks 1-20 | ||||||||||||||||||||||||||||
|
Select Excerpts from the Interview
Track 2
![]() DR LOVE: Can you discuss the use of induction chemotherapy followed
by chemoradiation therapy versus chemoradiation therapy alone in the
clinical setting?
DR LOVE: Can you discuss the use of induction chemotherapy followed
by chemoradiation therapy versus chemoradiation therapy alone in the
clinical setting?
![]() DR CURRAN: Until the last two or three years, induction chemotherapy had
fallen out of favor as not showing a substantial benefit. However, several trials,
most notably the Phase III TAX-324 trial (Posner 2007), have demonstrated
that a docetaxel-based induction regimen administered prior to radiation
therapy with a relatively nonintense concurrent chemotherapy regimen was
superior (2.3, 2.4).
DR CURRAN: Until the last two or three years, induction chemotherapy had
fallen out of favor as not showing a substantial benefit. However, several trials,
most notably the Phase III TAX-324 trial (Posner 2007), have demonstrated
that a docetaxel-based induction regimen administered prior to radiation
therapy with a relatively nonintense concurrent chemotherapy regimen was
superior (2.3, 2.4).
These findings made people realize that a role may exist for induction therapy. It should not, however, be administered instead of concurrent therapy, except to those patients for whom concurrent therapy is not feasible.
TAX-324 demonstrated that a benefit exists with docetaxel-based induction therapy (Posner 2007). The trial, however, doesn’t tell us whether it is superior to the more intense day-one chemoradiation therapy regimen used in the RTOG trial, which evaluates a docetaxel-based induction regimen prior to chemoradiation therapy versus a full-dose platinum and radiation therapy regimen on day one.
![]() DR LOVE: Has docetaxel been used as part of concurrent chemoradiation
therapy?
DR LOVE: Has docetaxel been used as part of concurrent chemoradiation
therapy?
![]() DR CURRAN: Yes, and there have been some promising results. It can be
administered on a weekly or an every three-week schedule. Either way, the
complete response rates and the progression-free survival rates appear to be
reasonable.
DR CURRAN: Yes, and there have been some promising results. It can be
administered on a weekly or an every three-week schedule. Either way, the
complete response rates and the progression-free survival rates appear to be
reasonable.
![]() DR LOVE: Can you discuss the side effects and toxicity associated with induction
therapy and chemoradiation therapy?
DR LOVE: Can you discuss the side effects and toxicity associated with induction
therapy and chemoradiation therapy?
![]() DR CURRAN: With induction therapy, the toxicities are the same as one
might see with any full-dose, every three-week taxane- or platinum-based
regimen — hematologic depression, stomatitis, risk of infection. Chemoradiation
therapy to the head and neck may be one of the most challenging cancer
therapies any patient goes through. In addition to the effects of the chemotherapy,
you also have the enhancement of the radiation effects by the chemotherapy.
DR CURRAN: With induction therapy, the toxicities are the same as one
might see with any full-dose, every three-week taxane- or platinum-based
regimen — hematologic depression, stomatitis, risk of infection. Chemoradiation
therapy to the head and neck may be one of the most challenging cancer
therapies any patient goes through. In addition to the effects of the chemotherapy,
you also have the enhancement of the radiation effects by the chemotherapy.
Grade III mucositis is a highly predictable side effect. Most patients who are going to receive radiation therapy to substantial portions of their mucosa need to have a PEG tube inserted prior to its initiation. It’s not a situation in which a feeding tube should be placed only after a certain amount of weight loss. It must be considered as part of the approach.
One concern early in the chemoradiation-therapy era was whether such intense regimens would result in radiation treatment delays, which we know are deleterious to tumor control. With the feeding tube and outstanding supportive care, most patients can get through radiation therapy without substantial treatment interruptions.
We find that providing the patient with a three-day weekend once or twice during a seven-week course, which doesn’t extend the treatment time, can be a godsend. This is not considered even a minor protocol violation if a patient happens to be involved in a clinical trial.
Track 5
![]() DR LOVE: Can you talk about the use of cetuximab in head and neck
cancer?
DR LOVE: Can you talk about the use of cetuximab in head and neck
cancer?
![]() DR CURRAN: Cetuximab is a monoclonal antibody that targets the epidermal
growth factor receptor (EGFR). Head and neck tumors that overexpress EGFR are associated with a worse prognosis in terms of recurrence and
survival. EGFR is a highly relevant target for head and neck cancer.
DR CURRAN: Cetuximab is a monoclonal antibody that targets the epidermal
growth factor receptor (EGFR). Head and neck tumors that overexpress EGFR are associated with a worse prognosis in terms of recurrence and
survival. EGFR is a highly relevant target for head and neck cancer.
Initially, cetuximab was studied in patients with head and neck cancer in a Phase I pilot study, which demonstrated that it was well tolerated, could be administered weekly during a course of radiation therapy and produced promising results (Robert 2001).
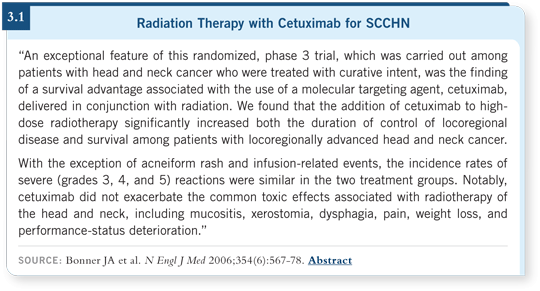
That trial led to an international Phase III study in which patients with Stage III or IV SCCHN were randomly assigned to treatment with radiation therapy alone or radiation therapy and weekly cetuximab (Bonner 2006).
A statistically significant improvement in survival and disease control was found for the patients in the cetuximab-containing arm (Bonner 2006; [3.1]). Interestingly, the magnitude of benefit was almost identical to what was seen when we evaluated radiation therapy with cisplatin versus radiation therapy alone.
The difference was we did not observe the enhancement of mucositis, stomatitis and locoregional toxicity that is associated with cisplatin, nor the hematologic toxicity (Bonner 2006; [3.1]). This trial allows us to consider another tool in our armamentarium for patients with head and neck cancer.
![]() DR LOVE: Can you talk more about the eligibility and design of that trial?
DR LOVE: Can you talk more about the eligibility and design of that trial?
![]() DR CURRAN: It was a one-to-one randomized Phase III design. Patients could
have Stage III or IV SCCHN. Some patients had high-risk tumors that were
resected. Others had unresected disease, but in general they did not have
bulky, bilateral, fixed lymph nodes.
DR CURRAN: It was a one-to-one randomized Phase III design. Patients could
have Stage III or IV SCCHN. Some patients had high-risk tumors that were
resected. Others had unresected disease, but in general they did not have
bulky, bilateral, fixed lymph nodes.
The overlap in eligibility between this study and the typical Phase III RTOG trial for Stage III/IV head and neck cancer was substantial, but this study included patients with slightly more intermediate- rather than high-risk locoregional head and neck cancer (Bonner 2006).
The median duration of overall survival was substantially higher — about a 50 percent increase. The absolute survival benefit at three years was 10 percent, but it was statistically significant, with a good hazard ratio and a p-value of 0.05 (Bonner 2006; [3.1]).
Track 7
![]() DR CURRAN: The question I hear frequently is, “Do I use chemoradiation
therapy or do I use chemotherapy with cetuximab? Or do I add cetuximab to
chemoradiation therapy?” Without having a final answer, the data we need are
on the safety of chemoradiation therapy with cetuximab.
DR CURRAN: The question I hear frequently is, “Do I use chemoradiation
therapy or do I use chemotherapy with cetuximab? Or do I add cetuximab to
chemoradiation therapy?” Without having a final answer, the data we need are
on the safety of chemoradiation therapy with cetuximab.
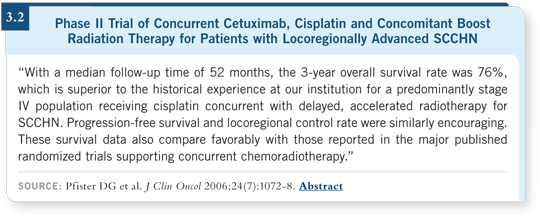
The most mature data come from a Phase II study at Memorial Sloan- Kettering in which patients received an altered fractionation regimen of radiation therapy, relatively aggressive concurrent chemotherapy and cetuximab (Pfister 2006).
A few years ago this study closed early due to concern over some early deaths (Pfister 2003). When a mature manuscript was published in the Journal of Clinical Oncology last year, we saw that those early deaths were probably not treatment or cetuximab related. The absolute survival, even accounting for those early deaths, and tumor control were outstanding (Pfister 2006; [3.2]).
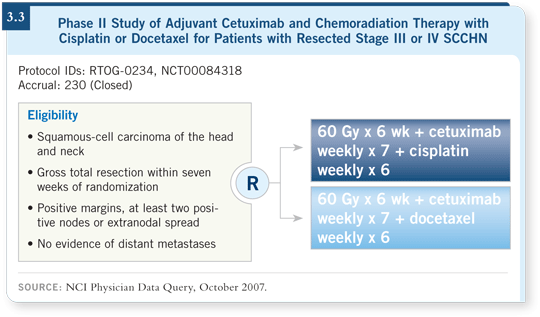
RTOG has completed accrual to a randomized Phase II study in which one arm receives radiation therapy, docetaxel and cetuximab (3.3), and the other arm receives radiation therapy, cisplatin and cetuximab. We have not observed any toxicity that made us feel we had to close the study early, but we don’t have the final results.
We have accrued about a third of the target number of patients in RTOG- 0522, which is evaluating radiation therapy/cisplatin versus radiation therapy/cisplatin/cetuximab (1.2, page 5). I am questioned about why we don’t have a third arm of radiation therapy/cetuximab.
We initially proposed that, but because that would have been equivalent to platinum and we would have been looking for a reduction in toxicity, it would have tripled the required number for accrual, which would have exceeded the feasible range of patient numbers.
For a patient who has a marginal indication for the addition of systemic therapy to radiation therapy but for whom you want to do more than radiation therapy, I recommend cetuximab. Among older patients, cetuximab is clearly well tolerated. For the patients with advanced disease, which would not have been included in the Bonner study, I would use chemotherapy if they could tolerate it. The patients in that middle zone are the challenging group. If a physician has a good discussion with the patient, and they want to use a regimen with Phase II but not Phase III data, I believe chemoradiation therapy and cetuximab is reasonable.
Track 9
![]() DR LOVE: What about patients who have head and neck cancer but also
have comorbidities such as alcohol abuse, malnutrition or heavy smoking
histories?
DR LOVE: What about patients who have head and neck cancer but also
have comorbidities such as alcohol abuse, malnutrition or heavy smoking
histories?
![]() DR CURRAN: Those are challenging medical and social issues. Clearly
continued use of tobacco has been associated with a poor outcome for patients
with this type of tumor. Anything that physicians can do to help patients with
pharmacologic and social tools to quit smoking before therapy begins will help
the patient. The same is true for alcohol abuse.
DR CURRAN: Those are challenging medical and social issues. Clearly
continued use of tobacco has been associated with a poor outcome for patients
with this type of tumor. Anything that physicians can do to help patients with
pharmacologic and social tools to quit smoking before therapy begins will help
the patient. The same is true for alcohol abuse.
Older gentlemen with all those problems are simply one facet of head and neck cancer. We’re seeing more people who don’t have a substantial history of tobacco or alcohol use. We’re seeing younger women and more demographic diversity. I can’t tell you why, but this is something we’re seeing in trial enrollment and elsewhere. We hope to see stronger advocacy on behalf of such patients. It’s a disease that needs advocacy as much as any disease because both the tumor and its treatment can be disabling and disfiguring. Any support we can give to patients who suffer from this will be tremendous.
My father passed away a couple years ago from head and neck cancer. Having him tell me he didn’t want to eat in public because of the disfigurement and the other problems brought home the issues we’re dealing with.
![]() DR LOVE: Did that change your perspective as a clinician or researcher?
DR LOVE: Did that change your perspective as a clinician or researcher?
![]() DR CURRAN: It was probably more of a personal nature than professional, but
certainly, seeing it from the point of view of the family does bring home the
issues. Seeing someone disabled, or his perception of disability, was probably
the strongest personal lesson — seeing him affected to the point of refusing to
go out in public because of his perception of his image. I believe other people
felt his appearance was less of a problem than he did himself — that was
probably the most poignant difficulty.
DR CURRAN: It was probably more of a personal nature than professional, but
certainly, seeing it from the point of view of the family does bring home the
issues. Seeing someone disabled, or his perception of disability, was probably
the strongest personal lesson — seeing him affected to the point of refusing to
go out in public because of his perception of his image. I believe other people
felt his appearance was less of a problem than he did himself — that was
probably the most poignant difficulty.
|
EDITOR
Neil Love, MD
INTERVIEWS
Edward S Kim, MD
- Select Publications
Marshall R Posner, MD
- Select Publications