

| Tracks 1-20 | ||||||||||||||||||||||||||||||||||||||||||
|
Select Excerpts from the Interview
Track 1
![]() DR LOVE: Can you discuss the emerging role of cetuximab in head and
neck cancer?
DR LOVE: Can you discuss the emerging role of cetuximab in head and
neck cancer?
![]() DR KIM: In preclinical models, cetuximab seemed very synergistic with
radiation therapy.
DR KIM: In preclinical models, cetuximab seemed very synergistic with
radiation therapy.
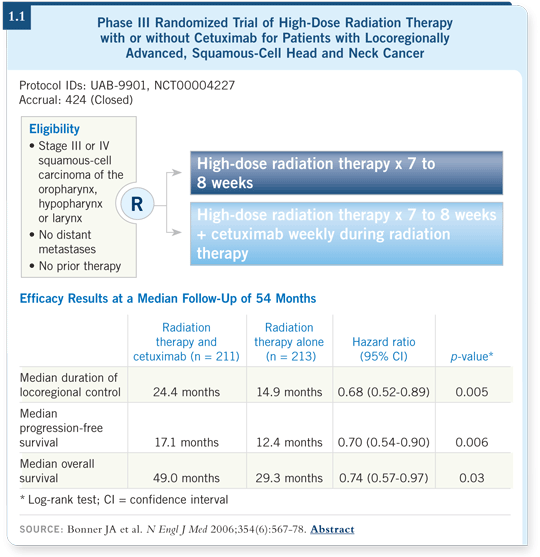
That led to a series of experiments and small trials, which culminated with Dr Bonner’s report published in The New England Journal of Medicine (Bonner 2006).
In that trial, the investigators evaluated cetuximab with radiation therapy versus radiation therapy alone for patients with locally advanced squamous-cell head and neck cancer. They demonstrated superiority with cetuximab (1.1).
The bonus was that, other than rash and hypersensitivity reactions, cetuximab added no toxicities to radiation therapy (Bonner 2006; [3.1, page 18]).
Tracks 3, 12
![]() DR LOVE: In a clinical setting, in which situations are you using
cetuximab for locally advanced disease?
DR LOVE: In a clinical setting, in which situations are you using
cetuximab for locally advanced disease?
![]() DR KIM: We tend to use cetuximab for patients who have minimal-bulk
disease — N1 disease of the oropharynx because the oropharynx hosts a
highly radiosensitive tumor — and for patients whom we don’t want to treat
with high-dose cisplatin.
DR KIM: We tend to use cetuximab for patients who have minimal-bulk
disease — N1 disease of the oropharynx because the oropharynx hosts a
highly radiosensitive tumor — and for patients whom we don’t want to treat
with high-dose cisplatin.
For patients who require chemoradiation therapy (ie, those with bulky neck nodes or primary tumors in unfavorable locations such as the hypopharynx or base of the tongue), concurrent chemoradiation therapy with high-dose cisplatin is still the answer.
RTOG-0522 is trying to determine whether cisplatin/cetuximab in combination with radiation therapy is better than radiation therapy and cisplatin (1.2).
![]() DR LOVE: What information do we have about chemoradiation therapy with
cetuximab, in terms of safety?
DR LOVE: What information do we have about chemoradiation therapy with
cetuximab, in terms of safety?
![]() DR KIM: Safety with cetuximab has been good. You don’t see exacerbations
of mucositis.
DR KIM: Safety with cetuximab has been good. You don’t see exacerbations
of mucositis.
Clearly we don’t have any myelosuppression, which is one of the big issues with cisplatin and results in many patients having to delay their chemotherapy. We try to plan three doses over a seven-week period, but frequently patients can receive only two doses.

The beauty of a drug like cetuximab is that you don’t have these side effects — it’s only the rash, and you’re using only eight doses, so the rash will go away. If you present that as a possible alternative treatment, then patients are amenable to it.
![]() DR LOVE: Is it your take that cetuximab doesn’t exacerbate the toxicities
associated with radiation therapy?
DR LOVE: Is it your take that cetuximab doesn’t exacerbate the toxicities
associated with radiation therapy?
![]() DR KIM: Absolutely. Many of us were worried that the rash might become a
problem in the radiated field especially.
DR KIM: Absolutely. Many of us were worried that the rash might become a
problem in the radiated field especially.
What’s ironic, as we’ve observed, is that patients who have undergone radiation therapy to an area and then receive cetuximab do not develop a rash in the radiated area.
![]() DR LOVE: What’s your impression in terms of quality of life in patients treated
with radiation therapy and cetuximab?
DR LOVE: What’s your impression in terms of quality of life in patients treated
with radiation therapy and cetuximab?
![]() DR KIM: As long as the rash is managed correctly and it’s not too severe, and
as long as patients don’t have a hypersensitivity reaction — which they can have
with taxanes and other drugs — they tolerate cetuximab beautifully. Cetuximab
is easy to administer. It is administered weekly, but patients don’t mind.
DR KIM: As long as the rash is managed correctly and it’s not too severe, and
as long as patients don’t have a hypersensitivity reaction — which they can have
with taxanes and other drugs — they tolerate cetuximab beautifully. Cetuximab
is easy to administer. It is administered weekly, but patients don’t mind.
Tracks 5-6
![]() DR LOVE: Can you discuss the TAX-323 clinical trial results?
DR LOVE: Can you discuss the TAX-323 clinical trial results?
![]() DR KIM: The study used four cycles of induction TPF (docetaxel/cisplatin/5-FU) followed by radiation therapy alone. The data for TPF were compelling
when compared to PF (cisplatin/5-FU). We learned that TPF, without the
expense of toxicities, was better than PF (Remenar 2006; [2.2, page 13]).
DR KIM: The study used four cycles of induction TPF (docetaxel/cisplatin/5-FU) followed by radiation therapy alone. The data for TPF were compelling
when compared to PF (cisplatin/5-FU). We learned that TPF, without the
expense of toxicities, was better than PF (Remenar 2006; [2.2, page 13]).
In the US, we don’t generally like to use four cycles of induction therapy. Three cycles are adequate, and we don’t want to delay the curative therapy, which is radiation therapy, much longer than that.
![]() DR LOVE: Which regimen do you use as induction therapy?
DR LOVE: Which regimen do you use as induction therapy?
![]() DR KIM: We know that PF, which was the historical standard, is inferior to
TPF without any tradeoff in side effects (Remenar 2006; Posner 2007).
DR KIM: We know that PF, which was the historical standard, is inferior to
TPF without any tradeoff in side effects (Remenar 2006; Posner 2007).
You’re not increasing side effects by adding docetaxel. Sometimes an occasional patient is unable to tolerate TPF. When I use TPF, I administer three cycles with growth factors.
I treat patients with two cycles and then restage the disease to make sure the tumor is shrinking. The patient knows before anybody else because TPF works well.
You can see the tumors shrink, which attests to the fact that squamous-cell tumors of the head and neck are particularly chemosensitive. After completing the third round of chemotherapy, I send the patient back to radiation therapy.
For patients with a poor cardiac status, multiple comorbidities or poor kidney function in whom we would like to start with induction therapy, we sometimes substitute carboplatin for cisplatin and drop the 5-FU.
Again, we have no data to support such a strategy. Many of us wondered whether cisplatin/docetaxel was as good as platinum/docetaxel/5-FU, but such a study hasn’t been and probably won’t ever be conducted.
For a fit patient, many times in the past, oncologists would pick a regimen such as platinum/5-FU or carboplatin/paclitaxel. Clearly we have a better regimen now, which is TPF. So if you decide to use induction therapy and the patient can tolerate it, TPF is the regimen you should use these days.
![]() DR LOVE: With TPF, how much of a problem is febrile neutropenia if you use
growth factors?
DR LOVE: With TPF, how much of a problem is febrile neutropenia if you use
growth factors?
![]() DR KIM: When I use growth factors, I don’t see too much febrile neutropenia.
Most of the side effects I’ve seen from TPF are based on 5-FU. Patients
will describe mucositis or hand-foot syndrome. Those types of toxicities have
compelled me to either hold 5-FU or decrease the dose. Generally, however, if
patients are faring well and you can keep their blood counts up, they tolerate
the regimen decently.
DR KIM: When I use growth factors, I don’t see too much febrile neutropenia.
Most of the side effects I’ve seen from TPF are based on 5-FU. Patients
will describe mucositis or hand-foot syndrome. Those types of toxicities have
compelled me to either hold 5-FU or decrease the dose. Generally, however, if
patients are faring well and you can keep their blood counts up, they tolerate
the regimen decently.
Track 15
![]() DR LOVE: Can you talk about the design and findings of the EXTREME
trial? How do you incorporate those results into your practice?
DR LOVE: Can you talk about the design and findings of the EXTREME
trial? How do you incorporate those results into your practice?
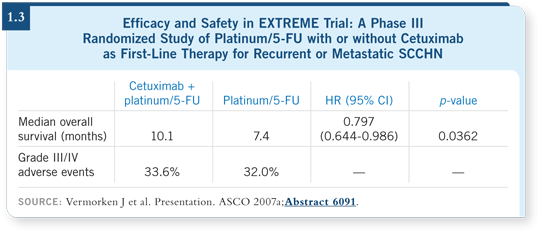
![]() DR KIM: The EXTREME study (Vermorken 2007a; [1.3]) was a nice proof-of-principle study, which validated EGFR-directed therapy even more firmly
than the Bonner study (Bonner 2006). Why do I say that?
DR KIM: The EXTREME study (Vermorken 2007a; [1.3]) was a nice proof-of-principle study, which validated EGFR-directed therapy even more firmly
than the Bonner study (Bonner 2006). Why do I say that?
When we talked about the Bonner study, we knew that radiosensitization worked with chemotherapy. We now also know that it works with EGFR-directed therapy. What we didn’t know was whether you could combine a drug that targets EGFR with chemotherapy and obtain a benefit.
Prior studies in recurrent metastatic head and neck cancer demonstrated that two drugs were no better than one drug. Dr Forastiere and Dr Jacobs have reported Intergroup and SWOG data to suggest that (Forastiere 1992; Jacobs 1992). So some people felt that methotrexate was a valid single-agent option in recurrent, metastatic disease.
Based on the old studies, that could not be disputed. Now, for the first time, we have proof that three drugs — a platinum/5-FU/cetuximab — are better than two drugs. We’ve never observed that before.
The addition of cetuximab did not seem to exacerbate side effects, and overall survival was improved by almost three months (Vermorken 2007a; [1.3]). I assume that cetuximab will now be incorporated into the first-line setting for recurrent, metastatic head and neck cancer.
Track 19
![]() DR LOVE: You coauthored a fascinating paper, with Tom Lynch and
Mario Lacouture, examining the issue of EGFR inhibitors and cutaneous
toxicity (Lynch 2007). Could you summarize some of your conclusions?
DR LOVE: You coauthored a fascinating paper, with Tom Lynch and
Mario Lacouture, examining the issue of EGFR inhibitors and cutaneous
toxicity (Lynch 2007). Could you summarize some of your conclusions?
![]() DR KIM: To me, it is such a shame when a patient has to have a therapy
stopped or held due to a rash.
DR KIM: To me, it is such a shame when a patient has to have a therapy
stopped or held due to a rash.
With patients who develop neutropenic fever, you obtain cultures and administer antibiotics. Then they come back and you treat them with the next dose of chemotherapy.
We have a patient who develops a rash, and we say, “We’re not going to use the drug any more.” I believe that’s a situation that can be avoided.
It requires a proactive approach from both the patient and the medical team to try to avoid the rash (1.4). The strategies we’ve developed at MD Anderson are similar to those Mario has developed.
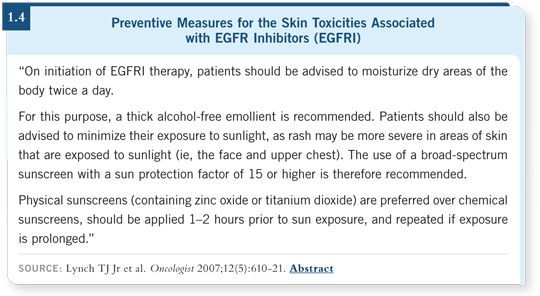
It’s about being proactive — not simply throwing creams on people and hoping the rash will go away. We want to move quickly to systemic therapies — antibiotics and/or steroids — to try to avoid any of these Grade III toxicities (1.4).
|
EDITOR
Neil Love, MD
INTERVIEWS
Edward S Kim, MD
- Select Publications
Marshall R Posner, MD
- Select Publications