
| Tracks 1-20 | ||||||||||||||||||||||||||||||||||||||||||
|
Select Excerpts from the Interview
Track 1
![]() DR LOVE: What are the current demographic trends for head and neck
cancer?
DR LOVE: What are the current demographic trends for head and neck
cancer?
![]() DR POSNER: The demographics are changing radically. In the past, most head
and neck cancer was associated with both heavy alcohol intake and smoking.
DR POSNER: The demographics are changing radically. In the past, most head
and neck cancer was associated with both heavy alcohol intake and smoking.
A few cases occurred in younger people, which were cryptic in their origin and tended to be devastating.
Of course, there’s an older group of people who have premalignant lesions that develop into cancer most likely related to environmental carcinogenesis or prior low-grade viral infections.
However, over the last decade and a half, there has been a marked increase in cancer at the base of the tongue and in the tonsils in young nonsmokers and nondrinkers.
The typical patient is between 45 and 55 years of age and has a two- to three-year history of smoking in college. They present with a painless mass in the neck that is found to be squamous cell or an identified primary in the tonsils or base of the tongue in association with a painless mass in the neck.
These are almost always HPV-16-positive. In fact, 60 to 70 percent of all tonsillar or base-of-tongue tumors in this country are being described as HPV-16-positive or associated with another carcinogenic HPV type found in the current vaccine.
Track 5
![]() DR LOVE: Can you talk about some of the key recent research developments
in this tumor?
DR LOVE: Can you talk about some of the key recent research developments
in this tumor?
![]() DR POSNER: The biggest development has been the use of targeted agents.
We now see cetuximab and EGFR antagonists coming into the clinic. The
radiation therapy/cetuximab data (Bonner 2006) are intriguing. It’s still difficult
to substitute that regimen for traditional chemoradiation therapy simply
because there’s only one good study.
DR POSNER: The biggest development has been the use of targeted agents.
We now see cetuximab and EGFR antagonists coming into the clinic. The
radiation therapy/cetuximab data (Bonner 2006) are intriguing. It’s still difficult
to substitute that regimen for traditional chemoradiation therapy simply
because there’s only one good study.
On the other hand, multiple studies show that cisplatin with radiation therapy is more effective than radiation therapy alone (Brizel 1998; Cooper 2004; Bernier 2004).
Over the next three or four years, cetuximab will be integrated into chemotherapy and chemoradiation therapy regimens in a more robust fashion, which I believe will be productive.
Interestingly enough, at ASCO 2007 the EORTC presented the EXTREME study of platinum/5-FU with cetuximab versus platinum/5-FU alone as first-line therapy for recurrent disease. The study demonstrated a significant improvement in overall survival, with the median duration increasing from 7.4 to 10.1 months (Vermorken 2007a; [1.3]).
This is the first time in head and neck cancer research that a combination of drugs was shown to be superior to any other combination for recurrent disease. It’s hard to demonstrate improvements in recurrent disease. It’s only when we evaluate these three-drug regimens — and particularly with a targeted noncross-resistant drug like cetuximab — that we see an additive effect.
Track 10
![]() DR LOVE: Can you review some of the key clinical trials evaluating
chemotherapy in the neoadjuvant setting?
DR LOVE: Can you review some of the key clinical trials evaluating
chemotherapy in the neoadjuvant setting?
![]() DR POSNER: The neoadjuvant setting is undergoing an evolution. For
the first time, we have established that a three-drug regimen, docetaxel/
platinum/5-FU, is superior to platinum/5-FU. As a result, survival has
been improved compared to platinum/5-FU, which had shown improved
survival compared to radiation therapy or surgery. Three trials have evaluated
docetaxel/platinum/5-FU (Calais 2006; Vermorken 2007b; Posner 2007).
DR POSNER: The neoadjuvant setting is undergoing an evolution. For
the first time, we have established that a three-drug regimen, docetaxel/
platinum/5-FU, is superior to platinum/5-FU. As a result, survival has
been improved compared to platinum/5-FU, which had shown improved
survival compared to radiation therapy or surgery. Three trials have evaluated
docetaxel/platinum/5-FU (Calais 2006; Vermorken 2007b; Posner 2007).
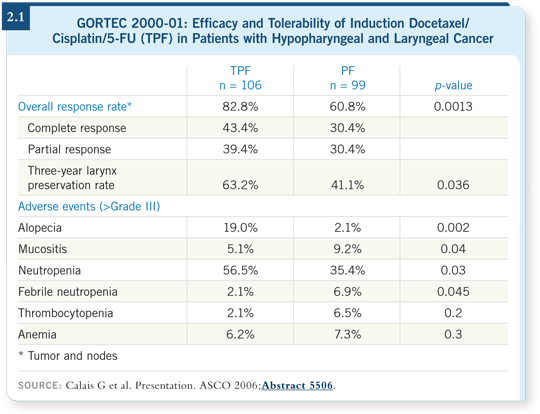
One trial by Calais, reported at ASCO 2006, was a larynx preservation trial for larynx and pyriform sinus cancers. A significant improvement was demonstrated in the laryngeal preservation rate for patients treated with three cycles of docetaxel/cisplatin/5-FU (TPF) compared to those treated with three cycles of cisplatin/5-FU (PF) as induction therapy (Calais 2006; [2.1]). Although it’s not clear at this time, there may be a survival advantage as well. However, that will depend on further analysis of the data as they mature.
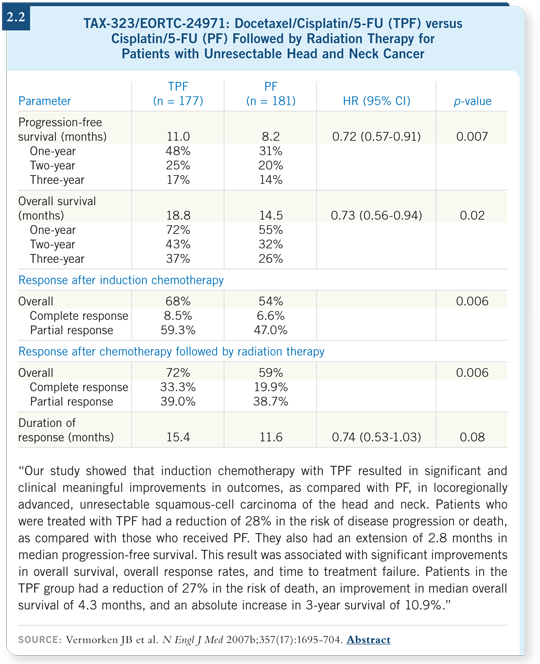
TAX-323/EORTC-24971 compared four cycles of TPF to four cycles of PF followed by radiation therapy for all patients with unresectable disease. Patients who received TPF had about a 30 percent reduction in mortality and an improvement in progression-free and overall survival (Vermorken 2007b; [2.2]).
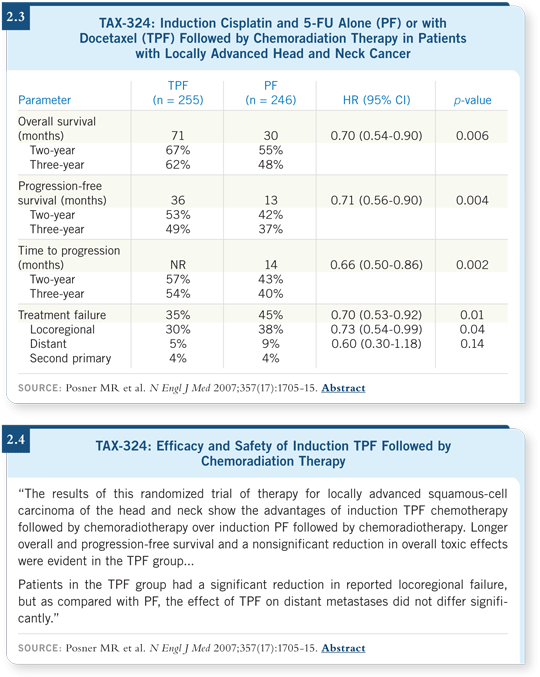
The third trial, TAX-324, was an international trial that included patients with resectable or unresectable disease. We administered three cycles of a more aggressive TPF regimen (docetaxel 75 mg/m2, cisplatin 100 mg/m2 and 5-FU 1,000 mg/m2/day, days 1-4, q3wk) and compared it to PF (cisplatin 100 mg/m2 and 5-FU 1,000 mg/m2/day, days 1-5, q3wk) (Posner 2007).
The induction chemotherapy was followed by concurrent chemoradiation therapy (five days per week) with bolus carboplatin (AUC 1.5 mg/mL) once a week (Posner 2007). We chose to do that to reduce the toxicity associated with the cisplatin that had been used in the past to improve the outcomes in terms of neurotoxicity and dehydration.
We saw a 30 percent reduction in mortality associated with TPF. Our three-year survival rate was 62 percent in the TPF arm versus 48 percent in the PF arm (Posner 2007; [2.3, 2.4]).
Track 16
![]() DR LOVE: What are the ongoing research strategies with the TKIs?
DR LOVE: What are the ongoing research strategies with the TKIs?
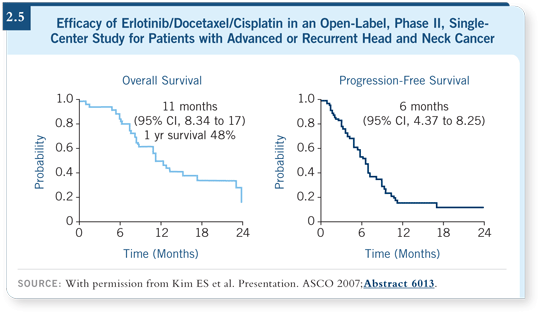
![]() DR POSNER: The MD Anderson data with cisplatin/docetaxel/erlotinib are
intriguing and potentially positive. Investigators conducted a Phase II trial, which is relatively mature, in which they administered cisplatin and docetaxel
at relatively high doses — 75 mg/m2 every three weeks for each — and
erlotinib at 150 mg daily. Patients received six cycles of treatment if they
continued to respond and then went on to maintenance erlotinib until they
had progressive disease or toxicity (Kim 2007). They had almost a 50 percent
one-year survival rate, which is good for recurrent disease. Four patients
(eight percent) had a complete response, 28 patients (58 percent) had a partial
response and 13 (25 percent) had stable disease (Kim 2007; [2.5]).
DR POSNER: The MD Anderson data with cisplatin/docetaxel/erlotinib are
intriguing and potentially positive. Investigators conducted a Phase II trial, which is relatively mature, in which they administered cisplatin and docetaxel
at relatively high doses — 75 mg/m2 every three weeks for each — and
erlotinib at 150 mg daily. Patients received six cycles of treatment if they
continued to respond and then went on to maintenance erlotinib until they
had progressive disease or toxicity (Kim 2007). They had almost a 50 percent
one-year survival rate, which is good for recurrent disease. Four patients
(eight percent) had a complete response, 28 patients (58 percent) had a partial
response and 13 (25 percent) had stable disease (Kim 2007; [2.5]).
|
EDITOR
Neil Love, MD
INTERVIEWS
Edward S Kim, MD
- Select Publications
Marshall R Posner, MD
- Select Publications