

| Tracks 1-16 | ||||||||||||||||||||||||||||||||||
|
Select Excerpts from the Interview
Track 1
![]() DR LOVE: Can you discuss the relationship between human papillomavirus
(HPV) infection and head and neck cancer?
DR LOVE: Can you discuss the relationship between human papillomavirus
(HPV) infection and head and neck cancer?
![]() DR HADDAD: HPV is the cause of the majority of cervical cancer cases.
We know, based on recent information, that HPV-16 is also a major cause
of oropharyngeal cancer (D’Souza 2007). This is specific for tumors on the tonsils and tongue base and is not applicable to cancer of the larynx or oral
cavity (Gillison 2000). These patients are typically young — in their thirties
or early forties — and are nonsmokers or nondrinkers. They present with
fairly advanced disease with large lymph node metastases in the neck and large
primaries on the tonsil or tongue base. The tumors are highly responsive to
chemotherapy and radiation therapy, and the prognosis for these patients with
HPV-positive oropharyngeal cancer is much better than for patients with
HPV-negative oropharyngeal cancer (Fakhry 2008).
DR HADDAD: HPV is the cause of the majority of cervical cancer cases.
We know, based on recent information, that HPV-16 is also a major cause
of oropharyngeal cancer (D’Souza 2007). This is specific for tumors on the tonsils and tongue base and is not applicable to cancer of the larynx or oral
cavity (Gillison 2000). These patients are typically young — in their thirties
or early forties — and are nonsmokers or nondrinkers. They present with
fairly advanced disease with large lymph node metastases in the neck and large
primaries on the tonsil or tongue base. The tumors are highly responsive to
chemotherapy and radiation therapy, and the prognosis for these patients with
HPV-positive oropharyngeal cancer is much better than for patients with
HPV-negative oropharyngeal cancer (Fakhry 2008).
Tracks 3, 6
![]() DR LOVE: Can you review the trial you presented at ASCO 2008 evaluating
cetuximab in combination with induction chemotherapy?
DR LOVE: Can you review the trial you presented at ASCO 2008 evaluating
cetuximab in combination with induction chemotherapy?
![]() DR HADDAD: We evaluated docetaxel, cisplatin and 5-FU (TPF) as induction
chemotherapy in combination with cetuximab for patients with locally
advanced head and neck cancer (Tishler 2008). It was a Phase I study in which
we escalated the dose of 5-FU. We used fixed doses of cisplatin, docetaxel and
cetuximab. The dose of 5-FU was escalated from 700 to 850 to 1,000 mg/m2 per day as a continuous infusion for four days. At a dose of 1,000 mg/m2 per
day, we ran into problems with gastrointestinal toxicity, probably from the
5-FU. So we de-escalated and declared 850 mg/m2 per day to be the
maximum tolerated dose.
DR HADDAD: We evaluated docetaxel, cisplatin and 5-FU (TPF) as induction
chemotherapy in combination with cetuximab for patients with locally
advanced head and neck cancer (Tishler 2008). It was a Phase I study in which
we escalated the dose of 5-FU. We used fixed doses of cisplatin, docetaxel and
cetuximab. The dose of 5-FU was escalated from 700 to 850 to 1,000 mg/m2 per day as a continuous infusion for four days. At a dose of 1,000 mg/m2 per
day, we ran into problems with gastrointestinal toxicity, probably from the
5-FU. So we de-escalated and declared 850 mg/m2 per day to be the
maximum tolerated dose.
We’ve enrolled only patients with fairly advanced disease, and so far we’ve had only one failure locally. All of the other patients continue to be in remission and are faring quite well. This was a Phase I/II trial, so at this point we will not draw many conclusions except that the combination is feasible and safe and should be studied further in Phase II and Phase III trials (Tishler 2008).
Track 4
![]() DR LOVE: What do we know about cetuximab in combination with
chemoradiation therapy?
DR LOVE: What do we know about cetuximab in combination with
chemoradiation therapy?
![]() DR HADDAD: The study that led to the approval of cetuximab in combination
with radiation therapy did not use chemotherapy (Bonner 2006). A remaining
question is how to combine cetuximab with concurrent chemoradiation therapy.
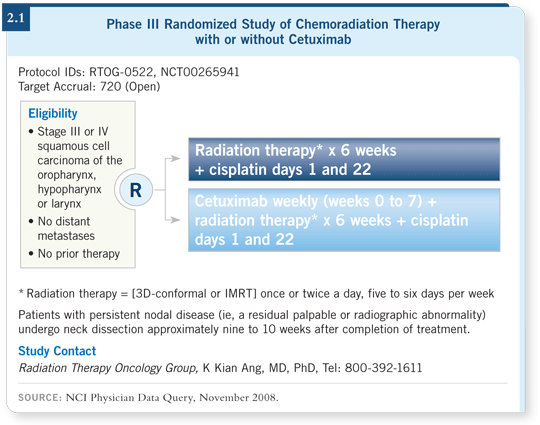
RTOG is currently performing a large Phase III trial (RTOG-0522) that will
enroll more than 700 patients and evaluate chemoradiation therapy with or
without cetuximab. The chemotherapy being used in that trial is cisplatin (2.1).
DR HADDAD: The study that led to the approval of cetuximab in combination
with radiation therapy did not use chemotherapy (Bonner 2006). A remaining
question is how to combine cetuximab with concurrent chemoradiation therapy.
RTOG is currently performing a large Phase III trial (RTOG-0522) that will
enroll more than 700 patients and evaluate chemoradiation therapy with or
without cetuximab. The chemotherapy being used in that trial is cisplatin (2.1).
Dr Pfister performed a Phase II study in which cisplatin, radiation therapy and cetuximab were combined, as RTOG is doing now. It was a small study that had to be stopped early because of an unexpected increase in the rate of toxicity. Even with those early toxicities, the overall results showed a promising rate of local control higher than 70 percent and, ultimately, cure for the patients who received these therapies (Pfister 2006).
Unfortunately, I believe a problem occurred with patient selection, and some of the patients enrolled in this trial died of toxicity. So the study was stopped early and could not be completed. The overall data, however, were promising enough for RTOG to consider their current randomized Phase III trial (RTOG-0522).
Track 8
![]() DR LOVE: What do you consider reasonable, evidence-based strategies
that can be used outside of a protocol setting for patients with locally
advanced head and neck cancer?
DR LOVE: What do you consider reasonable, evidence-based strategies
that can be used outside of a protocol setting for patients with locally
advanced head and neck cancer?
![]() DR HADDAD: For those patients in whom you perceive a high risk of distant
failure — those who have N3, N2b or N2c disease — the options would
include sequential therapy with induction chemotherapy followed by concurrent
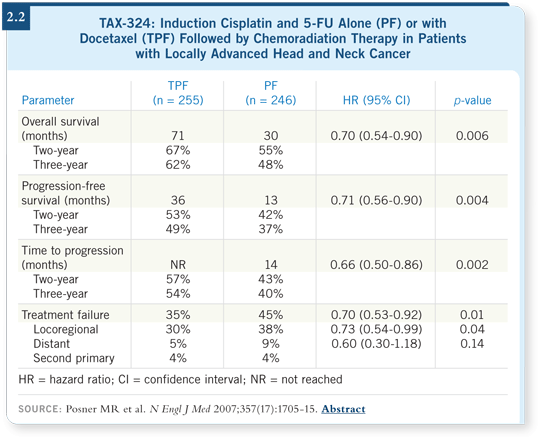
chemoradiation therapy. This is based on the TAX-324 study (Posner
2007; [2.2]). The other option is concurrent chemoradiation therapy with
bolus cisplatin administered every three weeks during radiation therapy. That
is considered by many to be the standard approach for locally advanced head
and neck cancer.
DR HADDAD: For those patients in whom you perceive a high risk of distant
failure — those who have N3, N2b or N2c disease — the options would
include sequential therapy with induction chemotherapy followed by concurrent
chemoradiation therapy. This is based on the TAX-324 study (Posner
2007; [2.2]). The other option is concurrent chemoradiation therapy with
bolus cisplatin administered every three weeks during radiation therapy. That
is considered by many to be the standard approach for locally advanced head
and neck cancer.
If the patient will not tolerate chemotherapy or refuses chemotherapy, I believe we have enough data to suggest a combination of cetuximab and radiation therapy. For that patient, the combination is superior to radiation therapy alone, and it does not necessarily increase the toxicity profile apart from the skin reactions (Bonner 2006).
|