

| Tracks 1-12 | ||||||||||||||||||||||||||
|
Select Excerpts from the Interview
Tracks 1-2
![]() DR LOVE: Can you discuss the background and results of ECOG-E3303?
DR LOVE: Can you discuss the background and results of ECOG-E3303?
![]() DR LANGER: We conceived of ECOG-E3303 six or seven years ago. Our goal
was to evaluate the addition of cetuximab to a standard regimen of chemoradiation
therapy including cisplatin for patients with unresectable squamous cell
carcinoma of the head and neck (Langer 2008; [1.1]).
DR LANGER: We conceived of ECOG-E3303 six or seven years ago. Our goal
was to evaluate the addition of cetuximab to a standard regimen of chemoradiation
therapy including cisplatin for patients with unresectable squamous cell
carcinoma of the head and neck (Langer 2008; [1.1]).
During the past 10 to 15 years, chemoradiation therapy had become the standard approach for locally advanced head and neck cancer.
In 2006, Jim Bonner published data demonstrating a survival advantage with cetuximab in combination with radiation therapy compared to radiation therapy alone (Bonner 2006; [3.1, page 13]). Cetuximab is the first targeted agent I’m aware of that’s been approved in the setting of definitive radiation therapy. A tremendous appetite exists to try to wed these two approaches by administering both chemoradiation therapy and cetuximab.
Years ago, Dave Adelstein conducted a landmark Phase III trial evaluating radiation therapy alone, full-dose radiation therapy with cisplatin and a split-course radiation therapy with 5-FU/cisplatin. Full-dose radiation therapy with cisplatin had the best outcome (Adelstein 2003). Our intention in ECOG-E3303 was to add cetuximab to that chemoradiation therapy schedule with a primary endpoint of progression-free survival. We accrued 69 patients, of whom 60 were clearly evaluable (Langer 2008). The demographics of the patients in ECOG-E3303 matched those in Dave Adelstein’s trial.
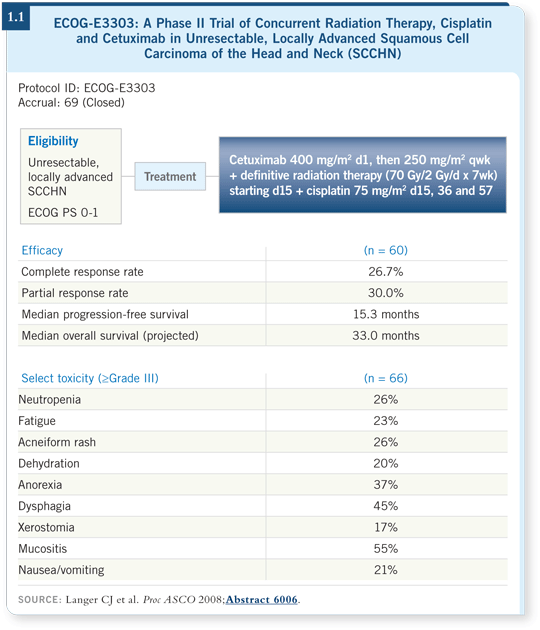
Overall, toxicity in ECOG-E3303 was reasonable, with two Grade V adverse events. We saw a fair amount of mucositis, dysphagia and, of course, acneiform rash. Twenty-six percent of the patients experienced a Grade III rash (Langer 2008; [1.1]).
The response rates don’t sound impressive, but we are living in the era of RECIST. I believe RECIST tends to downplay the response status. Overall, the response rate was 57 percent, and the median progression-free survival was 15 months, but that’s a fluid endpoint. Our median overall survival was 33 months, and we now have a two-year projected overall survival of 67 percent (Langer 2008; [1.1]).
In Adelstein’s study of radiation therapy versus chemoradiation therapy, the three-year overall survival for chemoradiation therapy with cisplatin was 37 percent. These results lend credence to the notion of adding cetuximab to chemoradiation therapy. In fact, RTOG is conducting RTOG-0522, a Phase III trial evaluating full-dose chemoradiation therapy with or without cetuximab (2.1).
Track 7
![]() DR LOVE: Would you consider radiation therapy in combination with
cetuximab in any situations, either with or without chemotherapy?
DR LOVE: Would you consider radiation therapy in combination with
cetuximab in any situations, either with or without chemotherapy?
![]() DR LANGER: Outside of a study, I feel uncomfortable administering all three
together. It’s still considered an experimental approach. I would certainly offer
participation in RTOG-0522 (2.1). If the patient declines — and probably 50
percent of those to whom I’ve offered it decline — I generally administer a
platinum agent alone with radiation therapy. If the patient is not fit enough
or another mitigating factor is present, such as age or comorbidity, I substitute
cetuximab for cisplatin, which would be identical to Bonner’s approach and
fits with evidence-based medicine.
DR LANGER: Outside of a study, I feel uncomfortable administering all three
together. It’s still considered an experimental approach. I would certainly offer
participation in RTOG-0522 (2.1). If the patient declines — and probably 50
percent of those to whom I’ve offered it decline — I generally administer a
platinum agent alone with radiation therapy. If the patient is not fit enough
or another mitigating factor is present, such as age or comorbidity, I substitute
cetuximab for cisplatin, which would be identical to Bonner’s approach and
fits with evidence-based medicine.
Track 8
![]() DR LOVE: What other research questions are being asked related to
cetuximab in head and neck cancer?
DR LOVE: What other research questions are being asked related to
cetuximab in head and neck cancer?
![]() DR LANGER: Ethan Argiris presented a study at ASCO 2008 that evaluated
cetuximab in combination with induction chemotherapy. He omitted 5-FU
but continued docetaxel and cisplatin. It was feasible, as we would expect
(Argiris 2008). It was a single-arm pilot trial, so we don’t have comparative
data, but I believe it’s a trend we’ll see. It won’t be docetaxel/cisplatin/5-FU
(TPF) alone. It may be TP with cetuximab. We may be using cetuximab to
substitute for a less effective, more toxic agent, such as 5-FU.
DR LANGER: Ethan Argiris presented a study at ASCO 2008 that evaluated
cetuximab in combination with induction chemotherapy. He omitted 5-FU
but continued docetaxel and cisplatin. It was feasible, as we would expect
(Argiris 2008). It was a single-arm pilot trial, so we don’t have comparative
data, but I believe it’s a trend we’ll see. It won’t be docetaxel/cisplatin/5-FU
(TPF) alone. It may be TP with cetuximab. We may be using cetuximab to
substitute for a less effective, more toxic agent, such as 5-FU.
RTOG-0522 will establish whether cetuximab adds to chemoradiation therapy. Until we have data from that trial, it remains an open question. Paul Harari has completed a Phase II trial in the adjuvant setting, in which cetuximab was administered with radiation therapy and either weekly cisplatin or weekly docetaxel (Harari 2007).
If those data seem promising, I can foresee a trial comparing a platinum agent with radiation therapy to a platinum agent with radiation therapy and cetuximab.
Finally, the EXTREME trial evaluated a platinum agent (carboplatin or cisplatin) and 5-FU with or without cetuximab in patients with recurrent or metastatic disease (Vermorken 2008; [4.1]).
Again, that trial showed a survival advantage with cetuximab as first-line therapy. I’m not aware of any other disease in which an agent has demonstrated its efficacy so globally, in nearly every setting.
|