

| Tracks 1-11 | ||||||||||||||||||||||||
|
Select Excerpts from the Interview
Track 2
![]() DR LOVE: Can you discuss the findings from RTOG-0129, comparing
conventional versus accelerated radiation therapy and concurrent cisplatin
for patients with Stage III or IV squamous cell carcinoma of the head and
neck?
DR LOVE: Can you discuss the findings from RTOG-0129, comparing
conventional versus accelerated radiation therapy and concurrent cisplatin
for patients with Stage III or IV squamous cell carcinoma of the head and
neck?
![]() DR ROSENTHAL: In this trial, all the patients received cisplatin, and they were
randomly assigned to receive either standard fractionation — five fractions
a week for seven weeks for a total dose of 70 Gray — or concomitant boost
treatment, which delivers approximately the same total dose, 72 Gray, in six
weeks (Ang 2007). The question is whether, in the setting of concurrent
chemotherapy, it is advantageous to accelerate the radiation therapy.
DR ROSENTHAL: In this trial, all the patients received cisplatin, and they were
randomly assigned to receive either standard fractionation — five fractions
a week for seven weeks for a total dose of 70 Gray — or concomitant boost
treatment, which delivers approximately the same total dose, 72 Gray, in six
weeks (Ang 2007). The question is whether, in the setting of concurrent
chemotherapy, it is advantageous to accelerate the radiation therapy.
The study closed three years ago, and the data are now maturing. We hope to have efficacy data in time for ASCO 2009. The preliminary safety data suggested that while some increase in mucositis and earlier acute toxicities occurred, the risk of some of the more worrisome consequential toxicities, such as dysphagia and longer-term feeding-tube dependency, is not increased (Ang 2007).
A study from Germany several years ago asked a similar question, but in that study all of the patients received accelerated fractionated radiation therapy and were randomly assigned to receive chemotherapy or not (Staar 2001). No improvement in survival outcomes occurred for the group that received both therapies, and almost half of the two-year survivors on that arm were feeding-tube dependent.
Therefore, I believe we need to be careful in using these aggressive chemoradiation therapy regimens until we have a clear signal of safety and efficacy. In my practice, I prefer to use concurrent chemotherapy with once daily radiation therapy until we see an advantage in accelerating radiation therapy.
Track 8
![]() DR LOVE: What’s your take on the role of cetuximab in the treatment of
head and neck cancer?
DR LOVE: What’s your take on the role of cetuximab in the treatment of
head and neck cancer?
![]() DR ROSENTHAL: The improvement in locoregional control and survival with
cetuximab when combined with radiation therapy (Bonner 2006; [3.1]) is
similar to the data seen when combining cytotoxic chemotherapy and radiation
therapy. Remarkably, the data for cetuximab in combination with radiation
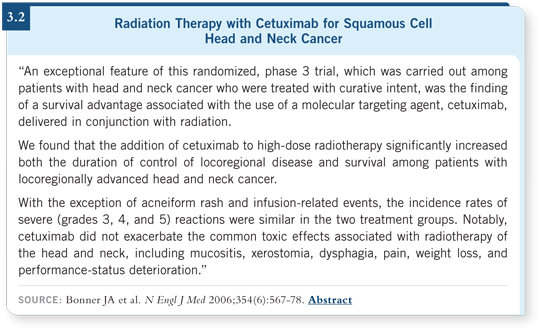
therapy showed no increase in mucositis, feeding-tube requirements or
Grade III dysphagia (Bonner 2006; [3.2]).
DR ROSENTHAL: The improvement in locoregional control and survival with
cetuximab when combined with radiation therapy (Bonner 2006; [3.1]) is
similar to the data seen when combining cytotoxic chemotherapy and radiation
therapy. Remarkably, the data for cetuximab in combination with radiation
therapy showed no increase in mucositis, feeding-tube requirements or
Grade III dysphagia (Bonner 2006; [3.2]).
The main scenario where I consider this agent off study now is for patients who are ineligible for cisplatin as a radiation sensitizer. Another involves the patient with a more borderline tumor, in which the physician may feel uncomfortable using radiation therapy alone but is hesitant to add the toxicities of concurrent chemotherapy.
Much debate has taken place regarding how we might use cetuximab instead of chemotherapy. We don’t have data directly comparing cetuximab to cisplatin as a radiation sensitizer. The results of the study comparing cetuximab with radiation therapy to radiation therapy alone (Bonner 2006; [3.1]) seem to be as good as the data from the trials in which chemotherapy was used.
![]() DR LOVE: Is there any situation in which you would use the combination of
cetuximab, chemotherapy and radiation therapy in clinical practice?
DR LOVE: Is there any situation in which you would use the combination of
cetuximab, chemotherapy and radiation therapy in clinical practice?
![]() DR ROSENTHAL: I don’t recommend it, and labeling specifically recommends
that it not be used. We typically don’t do it, even in our academic setting,
where sometimes we use more aggressive therapies.
DR ROSENTHAL: I don’t recommend it, and labeling specifically recommends
that it not be used. We typically don’t do it, even in our academic setting,
where sometimes we use more aggressive therapies.
One published study combined accelerated radiation therapy, chemotherapy and cetuximab, and it closed early due to untoward toxicities. However, positive outcomes were observed with the combination, and some of the toxicities probably could have been prevented had we known about some of the electrolyte-wasting properties, such as hypomagnesemia (Pfister 2006). Ultimately we might use this strategy, but I believe we need to wait for trials to validate its safety and efficacy.

Track 10
![]() DR LOVE: What about the role of induction chemotherapy?
DR LOVE: What about the role of induction chemotherapy?
![]() DR ROSENTHAL: Induction chemotherapy took hold after data from The
Department of Veterans Affairs Laryngeal Cancer Study Group suggested it
had a clear role in organ preservation (The Department of Veterans Affairs
Laryngeal Cancer Study Group 1991). Subsequent trials then showed that
despite an improvement in response, even complete response, it did not
ultimately affect locoregional control or survival.
DR ROSENTHAL: Induction chemotherapy took hold after data from The
Department of Veterans Affairs Laryngeal Cancer Study Group suggested it
had a clear role in organ preservation (The Department of Veterans Affairs
Laryngeal Cancer Study Group 1991). Subsequent trials then showed that
despite an improvement in response, even complete response, it did not
ultimately affect locoregional control or survival.
Recently we have seen trials evaluating more active drugs and combinations in the induction setting. Two Phase III trials — TAX-323 and TAX-324 — evaluated cisplatin/5-FU with or without docetaxel in patients who were to receive radiation therapy. Both trials reported improved survival with the addition of docetaxel (Vermorken 2007; Posner 2007; [2.2,).
|