

| Tracks 1-10 | ||||||||||||||||||||||
|
Select Excerpts from the Interview
Track 3
![]() DR LOVE: What are some of the major Phase III clinical trials that you
believe will shape the treatment of head and neck cancer in the next few
years?
DR LOVE: What are some of the major Phase III clinical trials that you
believe will shape the treatment of head and neck cancer in the next few
years?
![]() DR VOKES: The largest question in our minds at the University of Chicago
is that of the competing successful models — induction chemotherapy,
concurrent chemoradiation therapy and concurrent targeted therapy and
radiation therapy. Induction chemotherapy with the addition of docetaxel
to platinum/5-FU has been shown to be superior to platinum/5-FU alone
(Posner 2007; [2.2]). Induction chemotherapy, hence, has a role in the
combined-modality, curative-intent setting. Concomitant chemoradiation
therapy is superior to radiation therapy alone (Bourhis 2004), so that has a role. Recent evidence suggests that the addition of cetuximab to radiation
therapy also increases efficacy (Bonner 2006; [3.1]).
DR VOKES: The largest question in our minds at the University of Chicago
is that of the competing successful models — induction chemotherapy,
concurrent chemoradiation therapy and concurrent targeted therapy and
radiation therapy. Induction chemotherapy with the addition of docetaxel
to platinum/5-FU has been shown to be superior to platinum/5-FU alone
(Posner 2007; [2.2]). Induction chemotherapy, hence, has a role in the
combined-modality, curative-intent setting. Concomitant chemoradiation
therapy is superior to radiation therapy alone (Bourhis 2004), so that has a role. Recent evidence suggests that the addition of cetuximab to radiation
therapy also increases efficacy (Bonner 2006; [3.1]).
If you consider what these approaches do, differentially, you can postulate ways to combine them quite rationally. Concurrent chemoradiation, for example, will lead to better local control but not necessarily to better systemic control. Induction chemotherapy, on the other hand, I believe largely addresses micrometastatic systemic disease.
Hence, several randomized trials are underway. One trial (NCT00117572), which we are leading with Ezra Cohen as the principal investigator, is evaluating whether two cycles of induction chemotherapy administered before concurrent chemoradiation therapy can add further benefit compared to chemoradiation therapy alone. Marshall Posner and his group are leading a similar trial, and a European trial is also underway.
Track 5
![]() DR LOVE: In which situations, if any, do you integrate cetuximab into
the treatment for patients not enrolled in a study?
DR LOVE: In which situations, if any, do you integrate cetuximab into
the treatment for patients not enrolled in a study?
![]() DR VOKES: The addition of cetuximab to radiation therapy off protocol is
attractive because it is well tolerated and acts as a radiation sensitizer. We
would consider using cetuximab with radiation therapy for somewhat older
patients who may be frail and have comorbidities such that we would be
reluctant to administer chemotherapy. Similarly, we might consider cetuximab
with radiation therapy for patients with Stage III disease who were similar to
the patients included in the trial published by Bonner (Bonner 2006; [3.1]) — those who may be overtreated if they received induction chemotherapy
or one of the heavier chemoradiation therapy regimens.
DR VOKES: The addition of cetuximab to radiation therapy off protocol is
attractive because it is well tolerated and acts as a radiation sensitizer. We
would consider using cetuximab with radiation therapy for somewhat older
patients who may be frail and have comorbidities such that we would be
reluctant to administer chemotherapy. Similarly, we might consider cetuximab
with radiation therapy for patients with Stage III disease who were similar to
the patients included in the trial published by Bonner (Bonner 2006; [3.1]) — those who may be overtreated if they received induction chemotherapy
or one of the heavier chemoradiation therapy regimens.
![]() DR LOVE: Does cetuximab have a role in recurrent or metastatic disease?
DR LOVE: Does cetuximab have a role in recurrent or metastatic disease?
![]() DR VOKES: For a patient with unresectable recurrent disease or metastatic
disease, chemotherapy has been the standard for many years. Repeated trials
have compared agents or one combination to another.
DR VOKES: For a patient with unresectable recurrent disease or metastatic
disease, chemotherapy has been the standard for many years. Repeated trials
have compared agents or one combination to another.
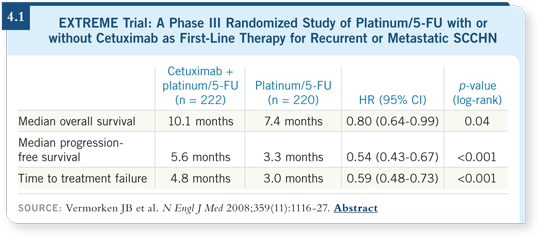
We never had a trial positive for survival until the EXTREME trial, which evaluated a platinum agent (cisplatin or carboplatin) and 5-FU with or without cetuximab (Vermorken 2008; [4.1]). Investigators reported an approximate two-month gain in overall and progression-free survival with the addition of cetuximab to chemotherapy. This was the first trial during my career as a head and neck oncologist that improved survival in the recurrent disease setting. So first-line chemotherapy for recurrent or metastatic disease, I believe, should include cetuximab.
Track 6
![]() DR LOVE: How do you approach treatment for patients with locally
advanced disease off study?
DR LOVE: How do you approach treatment for patients with locally
advanced disease off study?
![]() DR VOKES: Our first goal for that group of patients is cure, and a second
goal is organ preservation. Off protocol and based on many years of prospective
trials, we offer aggressive concurrent chemoradiation therapy as our first
approach. The regimen we use is quite intensive and involves administering
paclitaxel, infusional 5-FU and oral hydroxyurea with twice-daily radiation
therapy. Chemoradiation therapy is administered every other week for five
cycles for a total radiation dose of 75 Gray. Without adding induction chemotherapy,
we have reported long-term cure rates in the 60 to 70 percent range
in this group of patients with this regimen (Rosen 2003; Kies 2001).
DR VOKES: Our first goal for that group of patients is cure, and a second
goal is organ preservation. Off protocol and based on many years of prospective
trials, we offer aggressive concurrent chemoradiation therapy as our first
approach. The regimen we use is quite intensive and involves administering
paclitaxel, infusional 5-FU and oral hydroxyurea with twice-daily radiation
therapy. Chemoradiation therapy is administered every other week for five
cycles for a total radiation dose of 75 Gray. Without adding induction chemotherapy,
we have reported long-term cure rates in the 60 to 70 percent range
in this group of patients with this regimen (Rosen 2003; Kies 2001).
![]() DR LOVE: What about the role of induction chemotherapy off study?
DR LOVE: What about the role of induction chemotherapy off study?
![]() DR VOKES: I believe induction chemotherapy is conceptually attractive when
patients have advanced nodal disease. If the tumor has spread from the primary
and ipsilateral nodes, multiple lymph nodes are involved or there are bilateral
lymph nodes and an N3 node, we would worry greatly about that as a
predictor of widespread systemic micrometastatic disease. For that group of
patients I would think long and hard about using induction chemotherapy
because I believe they might benefit from systemic exposure to chemotherapy.
However, in a strictly scientific sense, that remains to be proven.
DR VOKES: I believe induction chemotherapy is conceptually attractive when
patients have advanced nodal disease. If the tumor has spread from the primary
and ipsilateral nodes, multiple lymph nodes are involved or there are bilateral
lymph nodes and an N3 node, we would worry greatly about that as a
predictor of widespread systemic micrometastatic disease. For that group of
patients I would think long and hard about using induction chemotherapy
because I believe they might benefit from systemic exposure to chemotherapy.
However, in a strictly scientific sense, that remains to be proven.
|